Mostrar o rexistro simple do ítem
Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial
| dc.contributor.author | Comín-Colet, Josep | |
| dc.contributor.author | Manito, Nicolás | |
| dc.contributor.author | Segovia-Cubero, Javier | |
| dc.contributor.author | Delgado Jiménez, Juan Francisco | |
| dc.contributor.author | García-Pinilla, José Manuel | |
| dc.contributor.author | Almenar, Luis | |
| dc.contributor.author | Crespo-Leiro, María Generosa | |
| dc.contributor.author | Sionis, Alessandro | |
| dc.contributor.author | Blasco, Teresa | |
| dc.contributor.author | Pascual Figal, Domingo A. | |
| dc.contributor.author | González-Vílchez, Francisco | |
| dc.contributor.author | Lambert Rodríguez, José Luis | |
| dc.contributor.author | Grau, María | |
| dc.contributor.author | Bruguera, Jordi | |
| dc.date.accessioned | 2018-07-05T12:05:39Z | |
| dc.date.issued | 2018 | |
| dc.identifier.citation | Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail. 2018;20(7):1128-1136 | es_ES |
| dc.identifier.issn | 1388-9842 | |
| dc.identifier.issn | 1879-0844 | |
| dc.identifier.uri | http://hdl.handle.net/2183/20855 | |
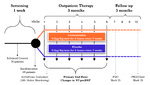
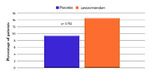
| dc.description.abstract | [Abstract] Aims. The LION‐HEART study was a multicentre, double‐blind, randomised, parallel‐group, placebo‐controlled trial evaluating the efficacy and safety of intravenous administration of intermittent doses of levosimendan in outpatients with advanced chronic heart failure. Methods and results. Sixty‐nine patients from 12 centres were randomly assigned at a 2:1 ratio to levosimendan or placebo groups, receiving treatment by a 6‐hour intravenous infusion (0.2 μg/kg/min without bolus) every 2 weeks for 12 weeks. The primary endpoint was the effect on serum concentrations of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) throughout the treatment period in comparison with placebo. Secondary endpoints included evaluation of safety, clinical events and health‐related quality of life (HRQoL). The area under the curve (AUC, pg.day/mL) of the levels of NT‐proBNP over time for patients who received levosimendan was significantly lower than for the placebo group (344 × 103 [95% Confidence Interval (CI) 283 × 103−404 × 103] vs. 535 × 103 [443 × 103−626 × 103], p = 0.003). In comparison with the placebo group, the patients on levosimendan experienced a reduction in the rate of heart failure hospitalisation (hazard ratio 0.25; 95% CI 0.11–0.56; P = 0.001). Patients on levosimendan were less likely to experience a clinically significant decline in HRQoL over time (P = 0.022). Adverse event rates were similar in the two treatment groups. Conclusions. In this small pilot study, intermittent administration of levosimendan to ambulatory patients with advanced systolic heart failure reduced plasma concentrations of NT‐proBNP, worsening of HRQoL and hospitalisation for heart failure. The efficacy and safety of this intervention should be confirmed in larger trials. | es_ES |
| dc.language.iso | eng | es_ES |
| dc.publisher | Wiley | es_ES |
| dc.relation.uri | https://doi.org/10.1002/ejhf.1145 | es_ES |
| dc.rights | This is the peer reviewed version of the article which has been published in final form at Wiley Online Library. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for self-archiving | es_ES |
| dc.subject | Levosimendan | es_ES |
| dc.subject | Pulsed infusions | es_ES |
| dc.subject | Outpatient setting | es_ES |
| dc.subject | Advanced heart failure | es_ES |
| dc.subject | Safety | es_ES |
| dc.subject | Natriuretic peptides | es_ES |
| dc.title | Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial | es_ES |
| dc.type | info:eu-repo/semantics/article | es_ES |
| dc.rights.access | info:eu-repo/semantics/embargoedAccess | es_ES |
| dc.date.embargoEndDate | 2019-02-06 | es_ES |
| dc.date.embargoLift | 2019-02-06 | |
| UDC.journalTitle | European Journal of Heart Failure | es_ES |
| UDC.volume | 20 | es_ES |
| UDC.issue | 7 | es_ES |
| UDC.startPage | 1128 | es_ES |
| UDC.endPage | 1136 | es_ES |
Ficheiros no ítem
Este ítem aparece na(s) seguinte(s) colección(s)
-
INIBIC-ICATC - Artigos [156]